Skip to content
AI-Driven Data Prediction Workflow for Compounds
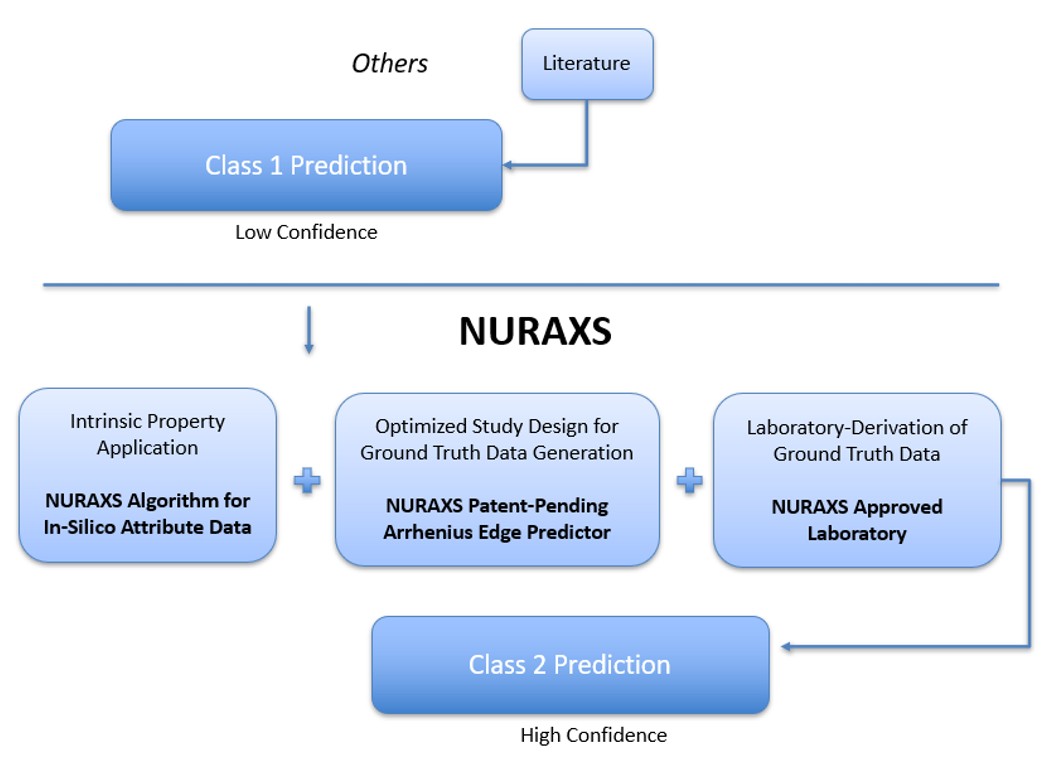
Data Inputs
- Scientific Literature
- Sponsor-Provided Product Data
- Laboratory-Generated Data (e.g., Valentia)
- GMP/GMP-like experimental results from AI-designed stress, stability, and accelerated studies.
- High-resolution data that can include chromatography, electrophoresis, cell-based bioassays, binding ELISA data, etc.
AI Model Design Loop
- Experiment Design
- AI proposes targeted experiments (stress testing, humidity/salt chamber studies, degradation kinetics).
- Execution in Lab
- Experiments performed using GMP/GMP-like equipment.
- Data collected in standardized, quality-controlled formats.
- Feedback to AI
- New data feeds back into the AI to refine prediction algorithms.
- Iterative Refinement
- Cycle continues until model convergence on high-confidence predictive patterns.
Data Integration Layer
- Normalization & Cleaning
- Standardizes formats across literature, sponsor, and lab data.
- Feature Extraction
- Identifies degradation pathways, kinetics, impurity formation, potency decay, packaging interactions.
- Multi-Source Fusion
- AI integrates heterogeneous datasets into a unified prediction model.
Prediction Engine
- Model Training & Refinement
- Uses machine learning (time-series, survival models, regression, deep learning).
- Critical Prediction Outputs
- Shelf-life projections (real-time & accelerated).
- Stability under varied storage conditions.
- Probabilistic confidence intervals for regulatory submission.
Validation & Reporting
- Cross-Validation
- Compare predictions against existing stability data and confirm robustness.
- GMP/GMP-like Compliance Layer
- Ensures outputs traceable, auditable, and regulatory acceptable.
- Deliverable
- Critical Prediction Report (e.g., accurate long-term shelf-life, risk of degradation).
- Visual dashboards and regulatory-ready outputs.